Abstract
Background Peripheral T-cell lymphoma (PTCL) is a rare and heterogeneous group of non-Hodgkin lymphoma and carries a poor prognosis. The four most common types are PTCL-NOS, angioimmunoblastic T cell lymphoma (AITL), and anaplastic large T cell lymphoma (ALCL) with or without ALK and together are grouped as nodal PTCL. In relapsed/refractory disease (r/r), the survival outcomes are poor and estimated to be approximately 13.7 months (Mak, Hamm et al. 2013). Hematopoietic cell transplantation (HCT) has been used to consolidate response and improve outcomes. However, the role of autologous HCT (auto HCT) and allogeneic HCT (allo HCT) remains controversial. Here we aim to compare the survival outcomes of auto HCT and allo HSCT in r/r PTCL.
Methods We retrospectively reviewed clinical data on 53 patients at Moffitt Cancer Center who developed r/r nodal PTCL and received HCT from 12/2007 to 12/2021. Patients who had auto HCT during the first remission were excluded. Physician discretion, donor availability, among others, were the factors that influenced transplant type allocation. Clinical data were abstracted in accordance with institutional review board-approved protocol. Patients were divided into two subgroups: cohort A) auto HCT and cohort B) allo HCT. The baseline clinical data of two cohorts were summarized using descriptive statistics and compared using Kruskal-Wallis tests for continuous variables and Chi-squared tests for categorical variables. Overall survival (OS) was calculated from the date of HCT to death or censored to the last follow-up. Median OS was calculated using the Kaplan Meier method and compared two cohorts with a log-rank test. Univariate and multivariate Cox proportional hazard (PH) using the backward elimination method were used to evaluate the association between OS and types of transplants.
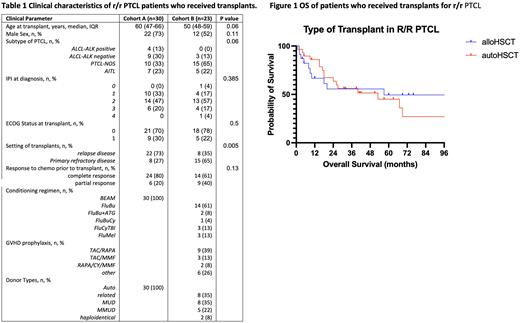
Results Out of 53 patients, 30 (57%) patients received auto HCT (cohort A), while 23 (43%) patients received allo HCT (cohort B). All patients achieved either partial response (PR) or complete response (CR) prior to transplant. The patients in allo cohort B were younger at the time of transplant (median age: 60 years vs. 50 years for cohort A and B, respectively, p=0.06), had a higher proportion of refractory disease (27% vs. 65% for cohort A and B, respectively, p=0.005), and had a higher proportion of PTCL-NOS (33% vs. 65% for cohort A and B, respectively, p=0.006) (table 1). ECOG status at transplant and IPI at the time of diagnosis were not significantly different between the two groups.
Median OS was 40 months in cohort A compared to 57 months in cohort B, however, no statistical difference was present (p=0.49) (Figure 1). In the univariate analysis, HR for allo HCT was 0.77 (p=0.5). When adjusted for ECOG status at transplant, histology subtypes, IPI score at diagnosis in the multivariate analysis, allo HCT was associated with prolonged OS with an HR of 0.41 (p=0.045).
At the study cutoff with a median follow-up of 57 months in cohort A, 18 patients died. Out of 12 patients with a known cause of death in cohort A, 7 patients (38%) died from relapse disease. With a median follow-up of 73 months in cohort B, 10 patients died. Out of 10 patients with a known cause of death, 2 patients (20%) died from the relapsed disease.
Conclusion Our data showed that allo HCT in r/r PTCL is associated with improved survival compared to auto HCT in multivariate analysis. Our result supports the use of allo HCT, especially in the younger/fit population and in refractory disease. Larger studies are needed to validate our results.
Disclosures
Lazaryan:AvroBio: Consultancy; Teladoc: Current equity holder in publicly-traded company; Sanofi: Consultancy; Humanigen: Consultancy; AmWel: Current equity holder in publicly-traded company. Shah:Servier: Other: grants and investigator-initiated trials; PeproMene Bio: Other: Steering committee; Autolus: Consultancy; Century Therapeutics: Consultancy; Adaptive: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; Acrotech: Consultancy; Jazz: Consultancy, Other: grants and investigator-initiated trials; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy, Other: grants and investigator-initiated trials; BMS/Celgene/Juno: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Saeed:Epizyme: Consultancy; Novartis: Consultancy; Morphosys: Honoraria. Jain:MyeloidTx: Consultancy; Incyte: Research Funding; BMS: Consultancy; Kite Pharma: Consultancy, Research Funding; Novartis: Consultancy. Liu:Sanofi: Speakers Bureau. Locke:Gerson Lehrman Group: Consultancy; Clinical Care Options Oncology: Other: Education or editorial role; Imedex: Other: Education or editorial role; Novartis: Research Funding; Allogene: Research Funding; Iovance: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding; Society for Immunotherapy of Cancer: Other: Education or editorial role; ASH: Other: Education or editorial role; Emerging Therapy Solutions: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Umoja: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Research Funding; BMS: Research Funding; National Cancer Institute: Research Funding; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Research Funding; BioPharma Communications CARE Education: Other: Education or editorial role; EcoR1: Consultancy; Cowen: Consultancy; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Calibr: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Education or editorial role; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; A2: Membership on an entity's Board of Directors or advisory committees. Pinilla Ibarz:Pharmacyclics: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; SecuraBio: Research Funding; Janssen Pharmaceuticals: Consultancy. Sokol:Dren Bio: Consultancy; Kyowa-Kirin: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal